Immune Regulation in Cancer and Stem Cell Transplantation
Arbeitsgruppe PD Dr. med. Poeck
Research Interests
Regenerative responses after allogeneic hematopoietic stem cell transplantation (allo-HSCT):
Our data suggest that endogenous cytosolic nucleic acid receptors including RIG-I-MAVS and cGAS-STING are involved in protecting epithelial integrity during GVHD and that therapeutic activation of RIG-I/MAVS can be exploited therapeutically to maintain epithelial barrier integrity and prevent GVHD. Our goal is to characterize the role of intestinal microbiota (bacterial / fungal) on the ISC compartment and immune system after damage induced by allo-HSCT and at understanding the innate immune pathways that can regulate barrier function after inflammatory tissue damage. We hypothesize that (i) endogenous bacteria and fungi will modulate the production of factors that are critical for intestinal homeostasis and self-renewal, and possibly support tissue regeneration by directly or indirectly activating of Lgr5+ ISC and/or its niche (Paneth cells) from intestinal pathology during pre-transplant conditioning or GVHD and (ii) microbiota (bacterial and/or fungal)-mediated tissue regeneration during GVHD is regulated by the indicated innate immune pathways. Our goal is to find ways to manipulate stem cell dynamics in order to improve prevention methods and therapies. Please find below a schematic overview of my current research projects related to ISCs:
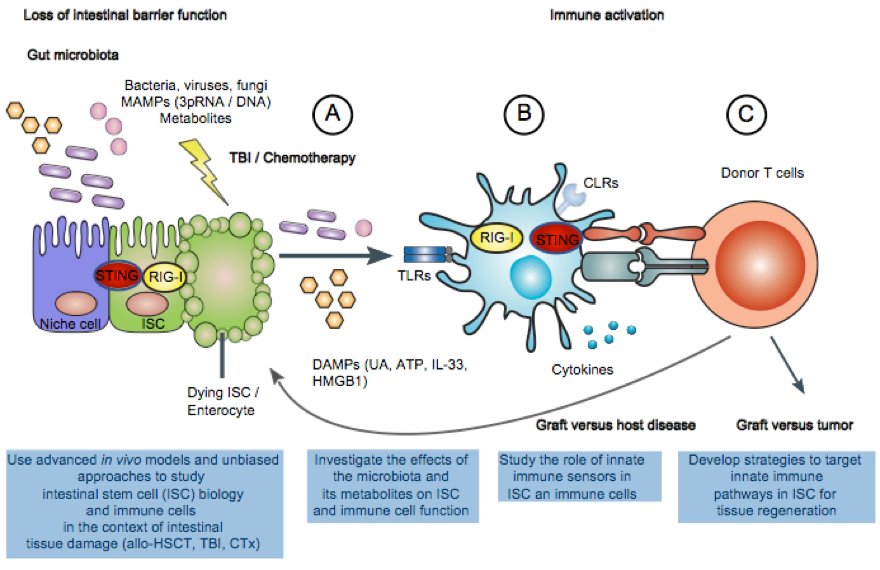
Schematic overview of the projects related to allogeneic hematopoietic stem cell transplantation (allo-HSCT):
Conditioning therapy-mediated damage to intestinal stem cells (ISCs) and other intestinal epithelial cells (IEC) leads to barrier function loss and translocation of otherwise luminal gut microbiota and release of danger-associated molecular patterns (DAMPs) (A). This triggers activation of recipient antigen presenting cells (APCs) that resisted the conditioning regime (B). Strong stimulation of recipient APCs results in enhanced cytokine production(B), priming and expansion of transplanted allogeneic donor T cells (C). Following an expansion phase, donor T cells can home to particular organs (gut, liver, skin) where destruction of allogeneic tissue of the recipient ultimately causes morbidity and mortality of GVHD. Thus, strategies to enhance ISC-mediated tissue regeneration are crucial for maintaining epithelial intestinal barrier function for the prevention of Graft versus host disease (GVHD) following conditioning therapy and allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Mechanisms of immune evasion and activation in tumor cells and their tumor microenvironment:
Our data identify tumor-intrinsic RIG-I signaling as a fundamental requirement for natural and therapy-mediated (immune checkpoint blockade (ICB)) antitumor immunity. Yet, malignant melanomas can evade IFN-I-dependent immune surveillance, implicating immuno-inhibitory mechanisms that may contribute to immune escape of melanoma and render these innate pathways insensitive to activation. Our goal is to define the contributions of aberrant (defective) tumor-intrinsic and host-dependent innate immune pathways during development and immunosurveillance of melanoma and other cancers as well as to identify the mechanisms and environmental factors (microbiome, metabolites, extracellular vesicles) that drive disease resistance and response to established immunotherapy protocols (i.e. ICB, radiation therapy, demethylating agents). We anticipate that our results will yield significant insights into improving outcomes of cancer patients as well as reveal molecular processes that are fundamental to immune –cancer cell communication. Please find below a schematic overview of my current research projects related to cancer resistance and immunotherapy:
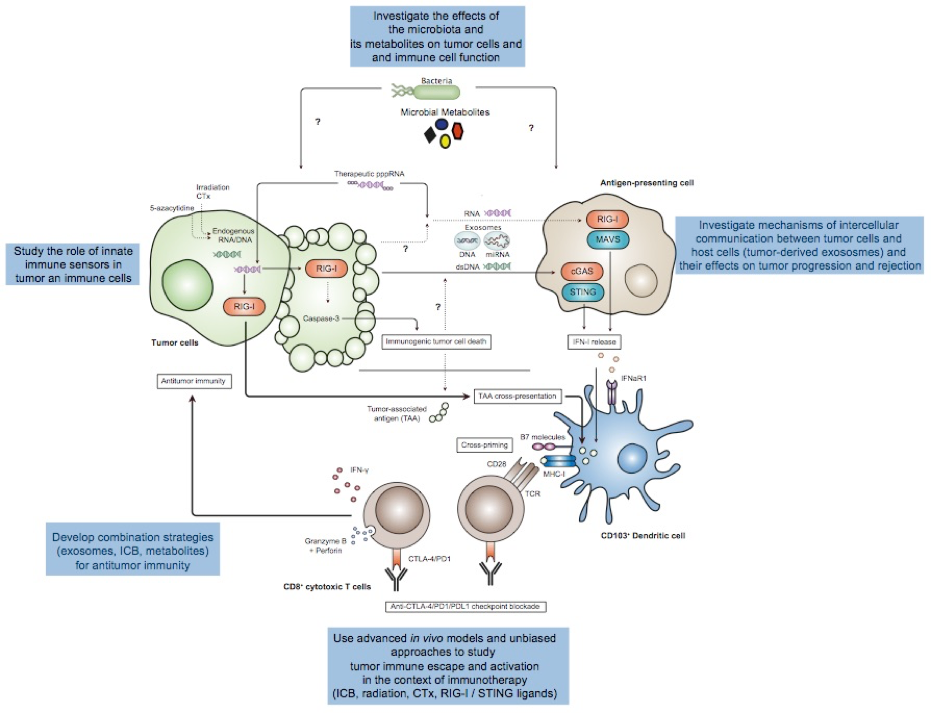
Schematic overview of the current projects related to cancer immunotherapy:
Genotoxic therapies and therapeutic nucleic acids can trigger potent IFN responses in antigen-presenting cells and simultaneously elicit immunogenic forms of cell death in cancer cells. Both mechanisms can lead to potent anti-tumor responses. Yet, cancers can also evade IFN-I-dependent immune surveillance, implicating immuno-inhibitory mechanisms that may contribute to immune escape of cancer and render these innate pathways insensitive to activation. Thus, better insights into the mechanisms that drive disease resistance and response to established immunotherapy protocols are urgently needed.
Bek S, Stritzke F, Wintges A, Nedelko T, Böhmer D.F.R, Fischer JC, Haas T, Poeck H* & Simon Heidegger* Targeting intrinsic RIG-I signaling turns melanoma cells into type I interferon-releasing cellular antitumor vaccines. (*These authors contributed equally). Oncoimmunology [2162-4011] Bek, Sarah J.:2019 S.:1 -9
Heidegger S, Kreppel D, Bscheider M, Stritzke F, Nedelko T, Wintges A, Bek S, Fischer JC, Graalman T, Kalinke U, Bassermann F, Haas T, Poeck H.RIG-I activating immunostimulatory RNA boosts the efficacy of anticancer vaccines and synergizes with checkpoint blockade. EBioMedicine. 2019 Mar 6. pii: S2352-3964(19)30135-5.
Fischer JC*, Bscheider M*, Eisenkolb G*, Lin CC, Wintges A, Otten V, Lindemans CA, Heidegger S, Rudelius M, Monette S, Porosnicu Rodriguez KA, Calafiore M, Liebermann S, Liu C, Lienenklaus S, Weiss S, Kalinke U, Ruland J, Peschel C, Shono Y, Docampo M, Velardi E, Jenq RR, Hanash AM, Dudakov JA, Haas T, van den Brink MRM*#, Poeck H.*# RIG-I/MAVS and STING signaling promote epithelial integrity during irradiation and immune-medited tissue injury. Sci Transl Med 2017;9. *#contributed equally
Fischer JC, Wintges A, Haas T, Poeck H. Assessment of mucosal integrity by quantifying neutrophil granulocyte influx in murine models of acute intestinal injury. Cell Immunol(link is external) 2017; 316:70-76.
Fischer JC, Otten V, Drees C, Schmickl M, Heidegger S, Beyaert G, van Loo G, Li XC, Peschel C, Schmidt-Supprian M, Haas T, Spoerl S, Poeck H. NF-κB regulator A20 modulates thymic regulatory T cell development. J Immunol. 2017;199:2356-2365.
Heidegger S, van den Brink MR, Haas T, Poeck H. The role of pattern-recognition receptors in graft-versus-host disease and graft-versus-leukemia after allogeneic stem cell transplantation. Front Immunol 2014;5:337.
Jankovic D, Ganesan J, Bscheider M, Stickel N, Weber FC, Guarda G, Follo M, Pfeifer D, Tardivel A, Ludigs K, Bouazzaoui A, Kerl K, Fischer JC, Haas T, Schmitt-Gräff A, Manoharan A, Müller L, Finke J, Martin S.F, Gorka O, Peschel C, Ruland J, Idzko M, Duyster J, Holler E, French LE, Poeck H*#, Contassot E*#, Zeiser R*#. The Nlrp3 inflammasome regulates acute graft-versus-host disease.(link is external) J Exp Med 2013;210:1899-910. *#contributed equally
Dann A*, Poeck H*, Croxford AL, Gaupp S, Kierdorf K, Knust M, Pfeifer D, Maihoefer C, Endres S, Kalinke U, Meuth SG, Wiendl H, Knobeloch KP, Akira S, Waisman A, Hartmann G, Prinz M. Cytosolic RIG-I-like helicases act as negative regulators of sterile inflammation in the CNS. Nat Neurosci 2011;15, 98-106. * contributed equally
Poeck H*, Bscheider M*, Gross O*, Finger K, Roth S, Rebsamen M, Hannesschläger N, Schlee M, Rothenfusser S, Barchet W, Kato H, Akira S, Inoue S, Endres S, Peschel C, Hartmann G*, Hornung V*, Ruland J*. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol. 2010;11:63-9. *contributed equally
Gross O*, Poeck H*, Bscheider M, Dostert C, Hannesschläger N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433-6. *contributed equally
Besch R*, Poeck H*, Hohenauer T, Senft D, Häcker G, Berking C, Hornung V, Endres S, Ruzicka T, Rothenfusser S, Hartmann G. Proapoptotic signalling by RIG-I and MDA-5 results in type I interferon independent apoptosis in melanoma. J Clin Invest 2009; 119, 2399-411. *contributed equally
Poeck H*, Besch R*, Maihoefer C, Renn M, Tormo D, Morskaya S, Kirschnek S, Gaffal E, Landsberg J, Hellmuth J, Schmidt A, Anz D, Bscheider M, Schwerd T, Berking C, Bourquin C, Kalinke U, Kremmer E, Kato H, Akira S, Meyers R, Häcker G, Neuenhahn M, Busch D, Ruland J, Rothenfusser S, Prinz M, Hornung V, Endres S, Tüting T, Hartmann G. 5’-triphosphate siRNA: turning gene-silencing and RIG-I activation against melanoma. Nat Med 2008; 4, 1256-63; *contributed equally
Principal Investigator
PD Dr. med. Hendrik Poeck
E-Mail(link sends e-mail)
Laboratory:
Center for Translational Cancer Research (TranslaTUM)
Lab Members
PostDoc Members and Senior Scientists
PD Dr. med. Simon Heidegger (MD), Senior Scientist (TUM, Department of Hematology and Oncology, since 2013, experiments running; Scholar Else Kröner-Fresenius-Stiftung Forschungskolleg München (since 2015). Best of Congress at DGHO 2015 (Talk: “Synergistische Wirkung von RIGI Agonisten und Checkpoint Blockade in der Tumor Immuntherapie”). Best of Congress at 32. Deutscher Krebskongress 2016 (Talk: “Activation of the cytosolic RNA receptor RIG-I in tumor and immune cells triggers efficient anti-tumor immunity and synergizes with checkpoint blockade”). Winner of the 3rd Immunotherapy of Cancer conference 2016 (ITOC3) Poster Award. Württembergischer Cancer Award (Nachwuchspreis) 2016. Habilitation (PD Dr. med) 04/2017. DKTK Best Highlight Talk of Young Scientists 2017. DGHO Young Investigator Award 2017. ASH Abstract Achievement Award 2017. ASCO Merit Award 2018. Scholar of EHA ASH TRTH Programm 2019
Erik Thiele-Orberg, (MD, Ph.D), Postdoctoral Fellow (TUM, Department of Hematology and Oncology, since 2017). Scholar Else Kröner-Fresenius-Stiftung Forschungskolleg (Start 11/ 2017). Darmkrebs-Präventionspreis 2017.
Graduate (PhD) students
Sascha Göttert, PhD Student (TUM Graduate School (TUM-GS) and Department of Hematology and Oncology, since 2018, experiments running)
Laura Joachim, PhD Student (TUM Graduate School (TUM-GS) and Department of Hematology and Oncology, since 2019, experiments running)
Medical Students (cand.med)
Gabriel Eisenkolb, cand. med. (TUM Graduate School (TUM-GS), “Promotionsprogramm translational medicine”, since 2014, experiments running)
Florian Stritzke, cand. med. (TUM Graduate School (TUM-GS), “Promotionsprogramm translational medicine”, since 2016, experiments running)
Stefan Enßle, cand. med. (TUM Graduate School (TUM-GS), “Promotionsprogramm translational medicine”, will start in 2019)
Research Assistant
Tatiana Nedelko (Ph.D, since 2018)
Lena Klostermeier (Biology student, since 2018)
Visiting Scientist
Esra Büyüközkan (MD, since 2018)
Former members
Julius Fischer, (MD), Postdoctoral Fellow
Michael Bscheider (MD), Postdoctoral Fellow
Chia-Ching Lin (PhD).; Postdoctoral Fellow
PD Dr. med. Silvia Spoerl (MD), Senior Scientist
Students (Ph.D and cand. med.)
Alexander Wintges, PhD
Sarah Bek, PhD
Julius Fischer, cand. med.
Vera Otten, cand. med.
Diana Kreppl, cand. med.
Michael Bscheider, cand. med.
Cornelius Maihöfer, cand. med.
Research Assistant
Martina Schmickl (Dipl. Ing. Biol)
Application
Written applications including CV are welcome and should be forwarded by e-mail(link sends e-mail) to Dr. med. Hendrik Poeck
Third party funding
EMBO (Young Investigator Programme)
European Hematology Association (EHA)
DKMS Foundation for Giving Life (DKMS)
SFB 1335 (German research foundation (DFG))
SFB 1371 (German research foundation (DFG))
Else-Kröner Fresenius-Stiftung (EKFS)
Promotionsprogramm „Translationale Medizin“ of the TU München



